Si and Mg self-diffusion coefficient in MgSiO3 perovskite (bridgimanite)
Purpose
- Use of single crystal MgSiO3 perovskite (bridgmanite)
-
- Technique of single crystal growth will be explained after.
- Simultaneous measurement of Mg and Si self diffusion coefficient
High pressure cell

Water content

An example of diffusion profile

Si and Mg self-diffusion coefficients

Comparison with previous studies

- The present DSi agree to the previous studies (Yamazaki et al., 2000; Dobson et al., 2008)
- Low DFe-Mg similar to DSi (Holzapfel et al.,2005)
- The present DMg shows clearer equivalency with DSi
Comparison of Mg diffusion

Explanation of low Mg diffusion

- A-site ion replaces closed packed O: A-site ion is fully surrounded by O
- No available unoccupied equivalent position for A-site ions
-
- 2/3 of 6-coordinated interstitials for B-site ions are unoccupied
Cluster diffusion

- Defect cluster (De Souza & Martin, 2004)
-
- Mg is surrounded by O
-
- Without O vacancy, Mg cannot move
- Identical DSi and DMg
-
- Si and Mg move together
Comparison with stishovite
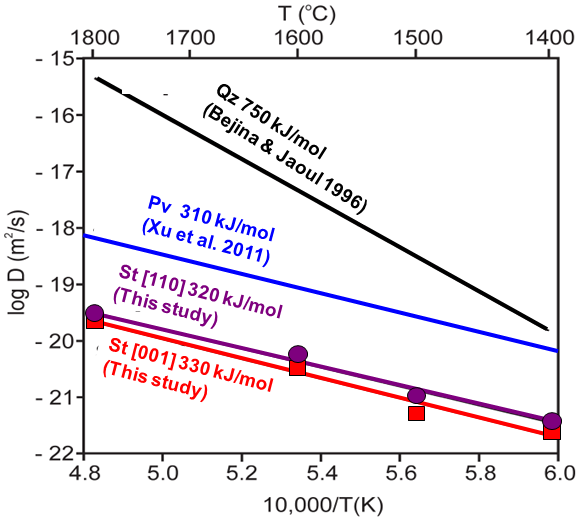
DSi in Pv > DSi in St
Why DSi in Pv >> DSi in St ?

- Oxygen defects are easier to form in Pv than St
-
- Anion vacancies are necessary for cations to hop.
-
- Cation : surrounded by Anion
- Oxygen packing in Pv is more distorted than that in St
- One O is bonded by 3 Si in St, whereas it is by 2 Si in Pv
- Mg-O bonding is weak in Pv due to too large distance
-
- orthorhombic distortion
DSi in cubic Pv << DSi in ortho Pv???
Comparison between Mg-Pv and Ca-Pv

CaPv is expected to have low DSi because of its non-distorted oxygen packing.
If so, subducted slab slab must be much harder than the surrounding mantle
Similar activation energy of DSi between Mg-perovskite and stishovite

- SiO6 octahedra in St is more distorted than those in Mg-Pv
-
- St : 3%
- Mg-Pv : 1 %
- Ea of St and Mg-Pv are similar
-
- about 300 kJ/mol
- Small distortion of SiO6 octahedra does not affect on DSi.
Summary
- Low DMg in Pv should be because Mg ions are fully surrounded by O ions in Pv structure.
- The equality of DSi and DMg in Pv implies the clustering of vacancies, and both cations move together.
- The Ea of DSi in Pv and St are similar.
- Small distortion of SiO6 octahedron does not affect on the local chemical environment of Si.
- Much higher DSi in Pv than St can be because of distortion of O packing in Pv due to Mg ions.
- Oxygen packing has large effects on Si diffusion, but the bonding environment has limited effects.
- The upper mantle viscosity should be almost constant.
Xu, J. S., D. Yamazaki, T. Katsura, X. P. Wu, P. Remmert, H. Yurimoto, and S. Chakraborty, Silicon and magnesium diffusion in a single crystal of MgSiO3 perovskite, J. Geophys. Res.-Solid Earth 116, doi:B12205, 10.1029/2011jb008444, 2011.
